
The McPin Patient and Public Involvement (PPI) panel have helped to shape the DIME study (Evaluation of a Ketogenic Diet in People with Treatment-resistant Depression: A proof-of-concept Trial).
This study aims to explore the therapeutic effects of a ketogenic diet on depression. This study offered a six-week programme of weekly dietitian counselling and provision of ketogenic meals, compared with an intervention involving similar dietetic contact time that promotes one extra portion of vegetables per day and switching to vegetable fat (placebo).
The PPI group provided feedback on the study design and adjustments were made accordingly.
Recruitment
The recruitment strategy should be more inclusive, accessible, and cover a broader range of gender and age groups, as well as diverse geographic areas. Furthermore, the content needs to be concise, informative, and easy to understand.
Response:
- After discussions with PPI members, a social media company was engaged for targeted study recruitment through targeted advertisements in real-time focused on underrepresented groups and modified as required ensuring a balanced representation across the UK of gender and age groups.
- A brief overview of eligibility criteria, study details, and participant expectations was included on the landing web page.
- Two versions of the landing web page were developed, and after polling 10 members from the PPI group, the most recommended page was published.
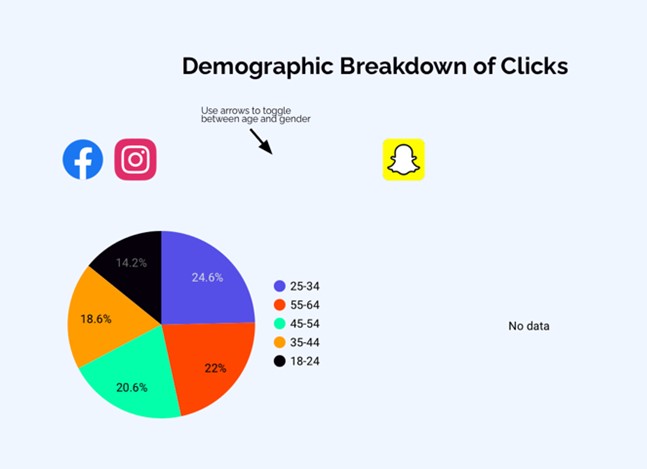
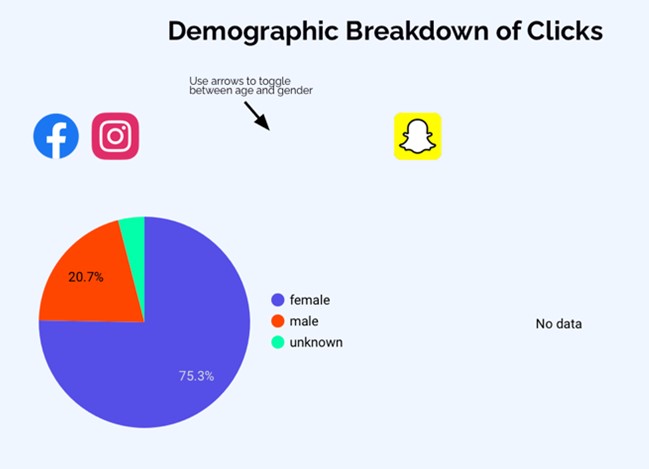
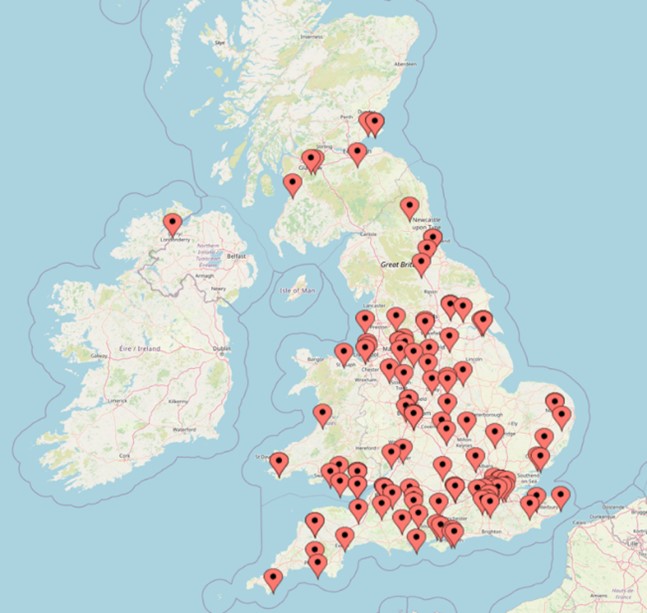
For the group receiving ketogenic diet meals
Some PPI members expressed concern that participants may feel hungry after the ready meals and suggested that extra snacks throughout the day are needed.
Response: We provided all participants with up to 3 snacks/day with various flavours and textures to cater to different preferences.
There were mixed feelings about offering prepared meals versus fresh food. Fresh food with a recipe card could enhance confidence and self-efficacy beyond the program, while some participants might prefer ready meals.
Response: We offered ready meals initially but also provided cooking and financial support for participants who prefer to cook their own keto meals.
For the meal options, cultural sensitivity is important.
Response:
- We asked each participant about their food preferences and adjusted the meals each week based on their input.
- While the food company didn’t offer culturally specific dishes, we advised participants during dietitian calls that they can change the flavour by adding their own spices and condiments.
The PPI panel expressed the importance of the content of leaflet materials and suggested having content to support barriers to adherence.
Response: We incorporated advice on seeking support from family members, planning for eating out, developing a relapse plan for special occasions, and adding a calendar to track progress.
For the control group
There is a need to better calculate the clinical effects of the intervention on depression.
Response:
We developed a placebo diet that promotes a healthier eating pattern, focusing on increasing vegetable intake and replacing saturated fats with unsaturated fats. This is a special design compared to other dietary interventions. This aims to be a plausible placebo dietary treatment for depression. There is no evidence that these manipulations will change mental health.
Study procedures
Sample kit collections need to be clear and easy to read
Response:
While PPI members fed back that it would be generally okay to undertake stool samples, they emphasised the need for clear explanations of their significance and usage. One member pointed out that the collection process should be clear to prevent misuse or self-harm. To address this, we provided simple instructions with pictures explaining the steps for saliva and stool samples. Mid-way through the trial, we noticed there were challenges pertaining to the saliva sample collection. We brought this issue to the PPI panel and panel members informed us that dry mouth is a very real symptom when on certain antidepressant medications. Through discussion, it was suggested to create a brief video in addition to the written instructions that discussed specific ways to ensure enough saliva was being collected for testing and how to package and repost the kits. The DIME team created a short instructional video in response to the PPI feedback and circulated the link to all participants. The panel also suggested that reminders would improve the completion rate for sample returns. Therefore, we implemented automatic reminders in our trial database, with a trial manager following up via phone call if kits were not returned after the third reminder.
GPs need to be notified about the study and participant involvement.
Response: We emailed their GPs about the study and the involvement of their patients.


